Naming organic molecules can seem like a daunting task, because lets face it, there are more rules than most of us care to learn. That being said, the aim of the International Union of Pure and Applied Chemistry is a noble one: to provide a system for providing every stable combination of atoms with a unique and insightful name. Fortunately, most of the biggest headaches in naming organic molecules come from edge-cases which you are unlikely to come across in a chemistry class. These following examples are intended to give you a look into the molecule-naming process.
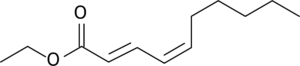
The First step in Naming any organic compound is to find the suffix functional group (if one exists). In this compound, There exists both an ester group, and two pairs of double bonded carbons. Since We don’t include double bonds in the suffix (along with triple bonds, and halogen groups), this compound is named as an ester.
Since all esters have a name of the form “alcohol-yl acid-oate”, we need to determine the alcohol and carboxylic acid that condensate to form this ester.
Naming the alcohol group is fairly easy, as it’s a simple two-carbon chain. Thus, this compound’s name will begin with ethyl.
The acid group of this compound is a little harder, but as it’s a single straight chain of 10 carbons (deca) with 2 double bonds (dien), we know that the root of its name will be decadienoate. The next step is to number the carbons of the double bonds, and record which ones are in the Cis (Z) or trans (E) configuration. In doing so, we want to choose the direction that minimizes the suffix group’s number, meaning that we have a trans double bond on carbon 2 and a cis double bond 4. As such, we can say that the compound’s name will end with (2E, 4Z)-decadienoate.
By putting these two together, we can now say that this compound is Ethyl (2E, 4Z)-decadienoate.
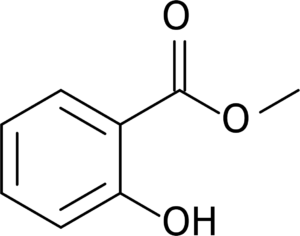
In this compound, we have either an ester, or an alcohol as our potential suffix group. Since the ester group is the more reactive of the two, we choose that one as our suffix.
As before, the alcohol portion of this ester is rather straightforward, Methyl.
For the acid, we must first find our parent chain. We can see that the carboxyl group is attached to a benzene ring. We call this benzene group with an additional carbon a Benzyl group. This Benzyl group is modified however, it has a hydroxyl group attached to it. To locate the hydroxyl group on the benzene ring, we assign the number 1 to the carbon that connects to the carboxyl, and chose the direction that minimizes the hydroxyl group’s distance from carbon 1. After all this, we can say that the acid part of this ester is 2-Hydroxybenzoic acid.
When we connect the two, we get Methyl 2-hydroxybenzoate, which is the preferred IUPAC name, however, this compound goes by a few more familiar names. Early botanists isolated samples of 2-hydroxybenzoic acid from willow trees, naming it Salicylic acid after the trees’ genus: Salix. As such, This compound can also be called Methyl Salicylate.
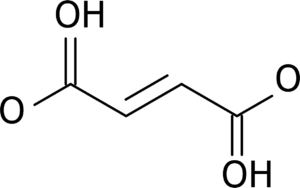
The suffix group of this compound isn’t too complicated, the most reactive groups are carboxylic acids, and there are 2 of them, making this a Dioic acid. The parent chain is 4 carbons long, and no matter which direction you start from, the carboxylic acids are on carbons 1 and 4, and the double bond starts on carbon 2.
Since a carbon can only hold a maximum of 4 bonds, any carbon participating in the carbon-carbon double bond can’t also have the 3 bonds necessary to be in a carboxyl group.
Therefore, if we state that double bond is on the 2nd and 3rd carbons, we don’t need to say that the carboxyls are on carbon’s 1 and 4, its Implied.
As such, This compound’s IUPAC name is But-2-enedioic acid.