Overview
Symmetrical molecules tend to follow basic structural shapes, such as linear, planar, tetrahedral, pyramidal, and bent. Some molecules also exhibit polarity, which gives them a distinct positive or negative charge.
VSEPR Theory
The shape of many small molecules can be predicted by the valence-shell electron pair repulsion theory or VSEPR for short. Electron pairs have the same negative charge, but the electrons share valances in molecular bonding. To compensate for the repulsion of like charges, the pairs of electrons in a molecular bond are as far apart from each other as they can be. This results in the distinctive shapes of small molecules, in order to preserve molecular bonding. Some atoms share electrons, and other electron pairs are not bonded.
Flat Molecular Shapes
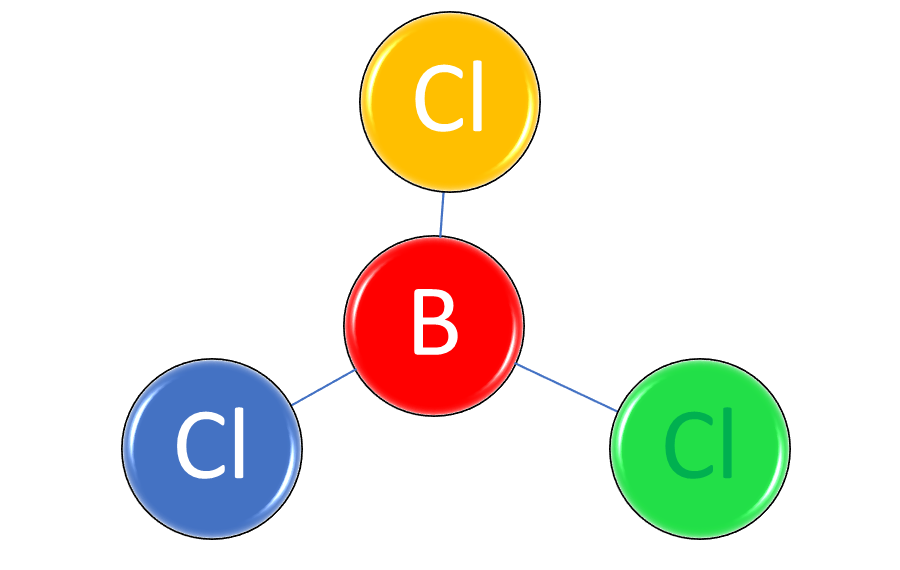
Some molecules are arranged with atoms in a straight line, such as carbon dioxide (CO2). The oxygen atoms are double-bonded with the carbon atom. The chemical formula for carbon dioxide doesn’t reflect its structure, but the type of strong atomic bonds does. All molecules that contain only two atoms have a linear bond. A trigonal planar molecule has three bonds in a Y-shape. In a molecule of boron trichloride (BCl3), each chlorine atom is bonded to the boron atom in the center.
Figure 1: Boron trichloride (BCl3) is an example of a trigonal planar molecule.
Three-Dimensional Molecular Shapes
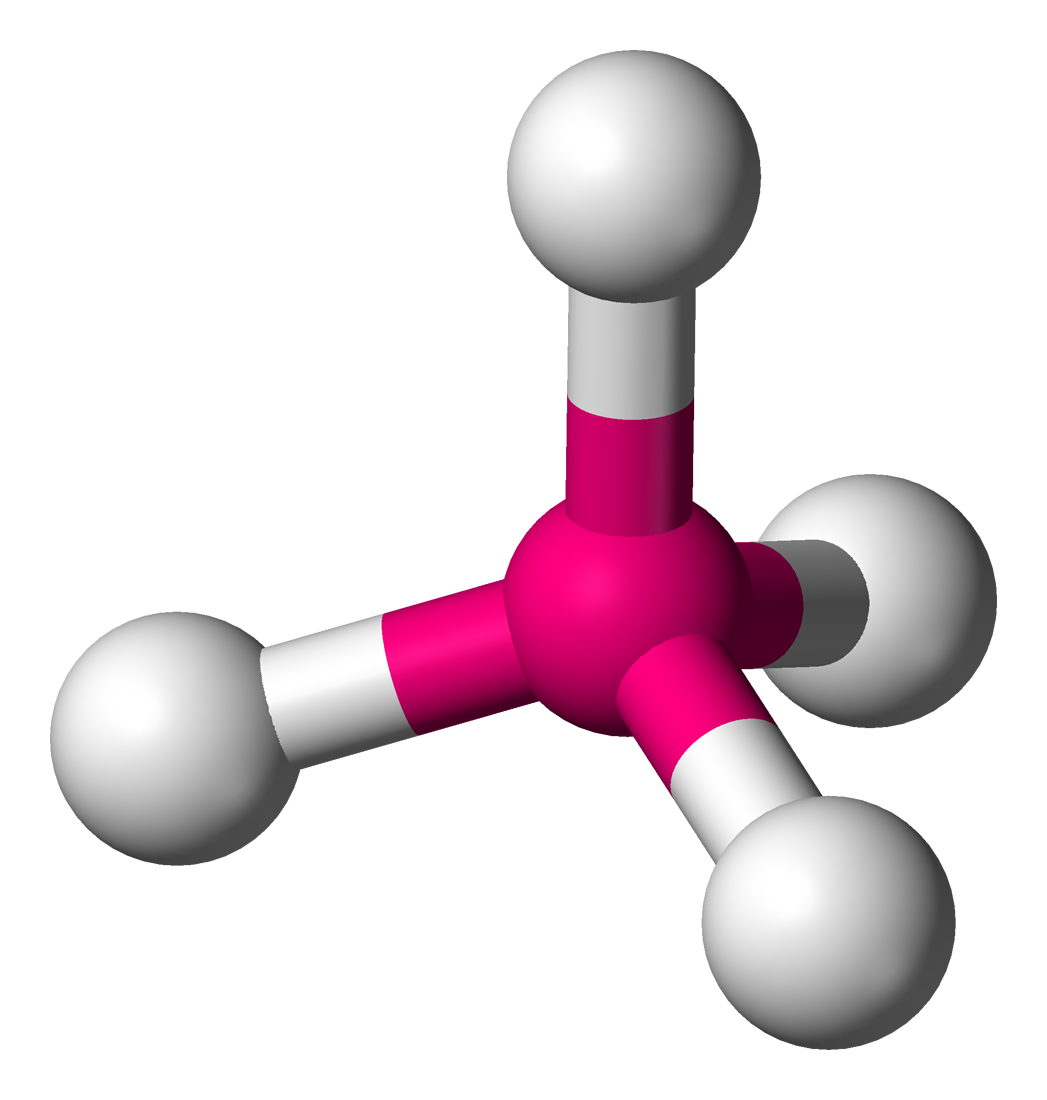
Some molecular shapes are a simple arrangement, but the atoms are arranged in three dimensions rather than the two dimensional flat molecular shapes. A molecule such as methane (CH4) has 4 atoms of hydrogen joined to one atom of carbon in the center. However, its atoms are not arranged in a two-dimensional square, but in a three-dimensional tetrahedral shape, similar to a tripod, called a tetrahedral shape. The atoms in ammonia (NH3) are arranged in a three-dimensional pyramid.
Figure 2: Three-dimensional tetrahedral molecular shape, such as methane (CH4). The carbon atom is the pink one in the center, surrounded by 4 white hydrogen atoms.
Other Shapes
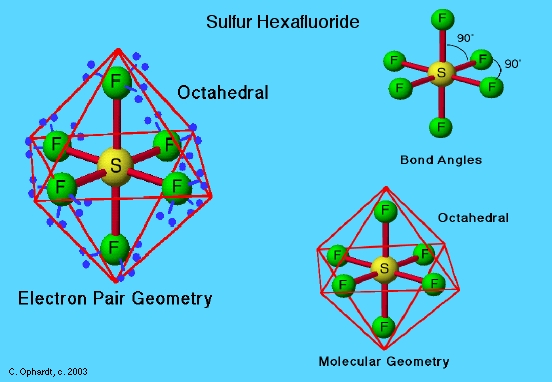
The hydrogen atoms in a water molecule (H2O) are joined to the oxygen atom in a bent shape, rather than a straight line. Other shapes include the T-shaped molecule chlorine trifluoride (ClF3), octahedral-shaped molecules such as sulfur hexafluoride (SF6), and other symmetrical and asymmetrical variations. Not all molecules have polarity, but those that do are negatively charged at one pole and positively charged at the other pole, because of the molecular bonds that have formed. Polar molecules are attracted by electrical charge, and line up in the charge.
Figure 3: Sulfur hexafluoride (SF6) is an example of an octahedral molecular shape.
Interested in chemistry tutoring services? Learn more about how we are assisting thousands of students each academic year.
SchoolTutoring Academy is the premier educational services company for K-12 and college students. We offer tutoring programs for students in K-12, AP classes, and college. To learn more about how we help parents and students in Smithfield, RI: visit Tutoring in Smithfield, RI