Overview
Reaction rates in chemistry are based on how fast that reactants turn into products. Some of the elements that affect reaction rates include the nature of the chemicals that act as reactants, the number and strength of the chemical bonds to be broken and formed, temperature, and the presence of catalysts.
Chemical Kinetics
Suppose a carton of potato salad is left in the refrigerator. The chemical processes that lead to its spoiling will take place very slowly. Leave that same carton in an unrefrigerated lunchbox, and it will become inedible much more quickly. Chemical kinetics is concerned with the speed of chemical reactions and the rates they take place. When the reactants are first brought into contact, there is a much higher proportion of reactant over product, then there is over time. The rate of reaction is determined experimentally, so the average rate of reaction is measured as the ratio of change in the amount or concentration of the reactant or the product with the change in time. (The symbol for change is ∆ Delta.)
Reaction Mechanisms
The reaction mechanism is a series of steps that lead from the beginning of the chemical reaction to the time when all reactants have been consumed, and have turned into products. It can be very detailed, as a description of steps that involve the molecules in the reactants as they are in the process of reacting. It goes beyond the chemical equation, to include intermediate steps. The intermediate steps proceed at their own rate until the reaction is complete.
Figure 1: A reaction mechanism is a listing of intermediate steps. In this example, there are 6 steps from the reactants to the products.
The Reaction Process
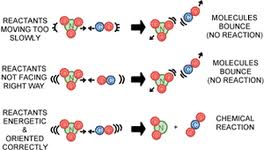
In order for chemical reactions to occur between substances, the molecules must come in contact with one another. This is known as collision theory. In order for a chemical reaction to begin, there must be enough energy to break chemical bonds within molecules. Every step of the reaction mechanism must continue in order for the reactants to be consumed.
Figure 2: Effective collisions result in chemical reactions. If there is not sufficient kinetic energy or the molecules are not in the right position, no reaction will occur.
Factors Affecting Reaction Rates
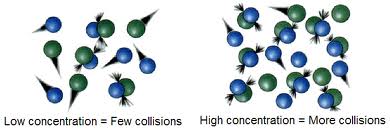
According to collision theory, effective collisions result in chemical reactions. There are a number of factors that affect reaction rates. The most rapid reactions occur between two gases, because a greater number of collisions occur among molecules moving rapidly. Similarly, more reactions can occur at higher temperatures, because the rapidly-moving molecules have more kinetic energy to collide.
Figure 3: Higher concentration is one of the factors that will increase reaction rate.
Interested in chemistry tutoring services? Learn more about how we are assisting thousands of students each academic year.
SchoolTutoring Academy is the premier educational services company for K-12 and college students. We offer tutoring programs for students in K-12, AP classes, and college. To learn more about how we help parents and students in Charleston, SC: visit Tutoring in Charleston, SC