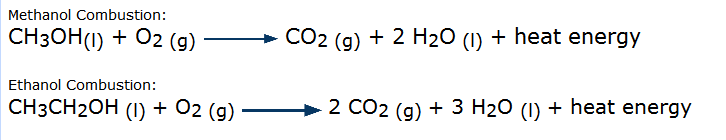Overview
Energy provides the capacity of matter to do work. Without it, there would be no chemical reactions or physical reactions and matter would be inert. It exists in forms such as potential energy and kinetic energy, and can be measured as heat.
Potential Energy
Potential energy is stored energy. Water behind a dam is stored as potential energy. Also, if a ball is located 5 feet above the ground, it has more potential energy than a ball that is at ground level. Gasoline carries potential energy to take part in chemical reactions within the internal combustion engine of an automobile. If an arrow is set on a bow and the bow is bent, the bent bow has potential energy stored.
|
Figure 1: As the archer draws the bow, the bow changes shape. The tension causes potential energy, which will be released as kinetic energy with the moving arrow. |
Kinetic Energy
Kinetic energy is the energy of any moving object. If the water behind the dam is released, the energy from the moving water will generate electricity from turbines. The ball located 5 feet above the ground will bounce higher and longer than one at ground level, as its potential energy is released. Similarly, the gasoline will combine with oxygen inside the internal combustion engine, allowing it to enter into chemical reactions that release its potential energy as it burns. As the vehicle moves, it carries kinetic energy. If a drawn bow is released, the nocked arrow will move through the air, converting potential energy to kinetic energy.
|
Figure 2: The kinetic energy generated by the moving water in this waterfall could be harnessed to drive turbines and produce hydroelectric power. |
Heat
Energy can be measured as heat. There are 2 units that measure heat: the joule and the calorie. The joule is the SI unit, and 4.184 joules (or J), equal 1 calorie. That is the amount of energy it takes to bring 1 g of water by 1 degree Celsius. Heat is involved in many chemical reactions, as a catalyst or as a product of the chemical reaction.
|
Figure 3: These balanced chemical reactions with two types of fuel and oxygen show how heat energy is released. |
Law of Conservation of Energy
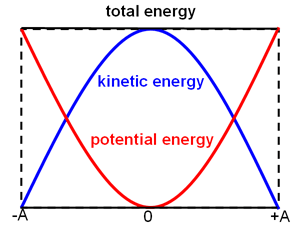
The Law of Conservation of Energy (the First Law of Thermodynamics) states that energy cannot be created or destroyed, but it can be transformed from one form to another. When fuel is burned, the heat energy that is released is part of the reaction. Similarly, the potential energy carried by a ball at the top of the hill is transformed into kinetic energy as the ball rolls down the hill.
Interested in chemistry tutoring services? Learn more about how we are assisting thousands of students each academic year.
SchoolTutoring Academy is the premier educational services company for K-12 and college students. We offer tutoring programs for students in K-12, AP classes, and college. To learn more about how we help parents and students in Missoula, MT: visit Tutoring in Missoula, MT