Overview
Many chemical reactions involve heat energy. They either release energy in the form of heat, or absorb energy in the form of heat from the environment.
Exothermic and Endothermic Reactions
Exothermic reactions generate heat. For example, liquid propane in a camp stove burns oxygen from the air to release heat in a chemical reaction as one of its products. Other reactions require heat to break chemical bonds. In order for water to be decomposed into hydrogen molecules and oxygen molecules, a great deal of heat is absorbed in an endothermic reaction.
Enthalpy Changes
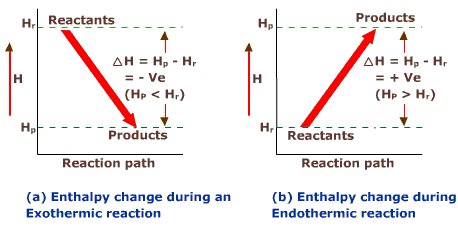
Both exothermic and endothermic changes can be expressed in terms of changes in enthalpy. It is closely related to energy, but takes into account the atmospheric or other pressure as a constant in the reaction. It is often represented by the variable ∆H, which equals heat products – heat reactants. (The delta∆ symbol means “change in”.) It is positive when the reaction is endothermic, because heat is added to the products, and it is negative when the reaction is exothermic, because heat is released. When chemical reactions that involve heat are balanced, enthalpy changes are also taken into account.
Figure 1: Exothermic and endothermic changes are changes in enthalpy.
Heat and Temperature
Heat and temperature are related concepts, but they are not exact synonyms. An exothermic reaction releases heat, so the temperature of the surrounding area increases. The size of that increase (or the ∆H) depends on the amount of heat that is released and on the heat capacity of the object in the surroundings. Suppose a beach fire is burning on the sand next to the bay. It will probably not increase the temperature of the bay, but it will boil the water in a teakettle close to it. The same amount of heat is released by the beach fire, but it affects the water in the teakettle more than the volume of water in the bay. The smaller volume of water in the teakettle has a smaller heat capacity, and heats more quickly.
Figure 2: The flame from a campfire creates chemical changes in the marshmallow as it toasts.
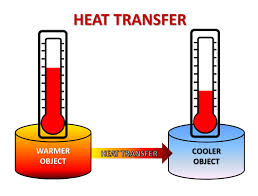
At one time, heat was thought to be released as a separate substance that was somehow contained in particles of matter. However, that theory did not explain many of the ways that heat is produced. Scientists now believe that heat is produced by the kinetic energy of moving molecules. Transfer of kinetic energy from hotter objects to colder objects produces heat and many other effects.
Figure 3: Kinetic energy is transferred from hotter objects to colder objects.
Interested in chemistry tutoring services? Learn more about how we are assisting thousands of students each academic year.
SchoolTutoring Academy is the premier educational services company for K-12 and college students. We offer tutoring programs for students in K-12, AP classes, and college. To learn more about how we help parents and students in Bethlehem, PA: visit Tutoring in Bethlehem, PA