Overview
A chemical reaction can be described by discussing the chemical substances at all steps. It is more efficient to use a chemical equation than words, and there are agreed-upon conventions to summarize the reaction, the substances that take part in it, the products, and the amounts.
Chemical Reaction
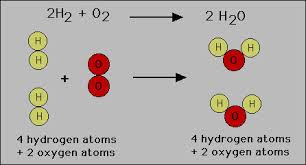
A chemical reaction starts with substances (often elements or compounds) that take part in it. They are called the reactants. Something happens to cause a change, and some part of the reactants are rearranged. Chemical bonds are formed, reformed, and broken, and new types of substances are made. Those new substances are the products of the reaction. For example, two atoms of hydrogen for every atom of oxygen combine to form water, so that 2H2 + O2→ 2 H2O.
Figure 1: Equation for the chemical reaction between hydrogen and oxygen to produce water.
Common Symbols
The symbols for the elements are taken from the periodic table, such as H for hydrogen, O for oxygen, Au for gold, K for potassium, and so on. The reactants are on the left of the equation, while the products are on the right. They are separated by an arrow (→) that points in the direction the reaction is taking. If the arrow is double and points in both directions (D) that means that the reactants and the products are in equilibrium. Heat is represented by the delta sign (∆) placed above the arrows. If a gas is released, it is shown by an arrow pointing upward (↑), and if a solid is precipitated, an arrow points downward (↓). If substances are added during a reaction, a plus sign is placed between the substances.
Figure 2: Methane and oxygen combine to form carbon dioxide and water.
Chemical Formulas Are in Context
Chemical formulas for a particular compound give a great deal of information that is subject to interpretation. The chemical formula for water is H2O. That can mean that there are 2 hydrogen atoms for every oxygen atom, one molecule of water, 1 mole (abbreviated mol) of water, 1 molar mass of water, 6.022 x 1023 molecules of water (Avogadro’s number), or 18.02 grams of water.
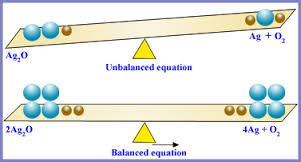
A Balanced Equation
When a chemical equation is balanced, it is clear what substances are the reactants, which are the products, how much of each substance is involved, as well as their relationship to each other, and the steps that occur during the reaction. Suppose that hydrogen gas reacts with chlorine gas to produce hydrogen chloride, which is also a gas. In symbol form, H2 (g) + Cl2(g) → 2 HCl (g).
Figure 3: When a chemical equation is balanced, it has the right amount of reactants and products on both sides.
Interested in chemistry tutoring services? Learn more about how we are assisting thousands of students each academic year.
SchoolTutoring Academy is the premier educational services company for K-12 and college students. We offer tutoring programs for students in K-12, AP classes, and college. To learn more about how we help parents and students in Gastonia, NC: visit Tutoring in Gastonia, NC