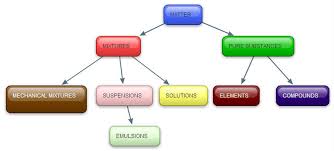
Overview
Substances occur in elements or compounds, while mixtures can be any combination of one or more elements or compounds. Mixtures may occur as solutions or heterogeneous combinations.
Elements and Compounds
Elements and compounds are known as pure substances. A molecule of one of the elements or compounds only contains that substance. For example, a molecule of oxygen, O2, only contains oxygen. A molecule of water, H2O, only contains 2 atoms of hydrogen to one atom of oxygen.
Homogenous or Heterogeneous
Pure substances are always homogeneous. The ideal pure substance contains molecules of only that substance, and nothing else. Mixtures may be homogeneous (if they are solutions) or heterogeneous. If they are heterogeneous, that means that different substances are throughout the mixture. For example, sand is a mixture that contains many different silicates, other types of rocks, organic debris such as particles of shells, and miscellaneous other items.
Solutions
Solutions are mixtures that are homogenous. Dissolve a teaspoon of sugar in a cup of hot water. Both the sugar molecules and the water molecules exist separately, as can be demonstrated if the water is allowed to evaporate. Homogenized milk is a suspension of fat globules in liquid. By definition, the milkfat globules are evenly distributed throughout the liquid rather than being allowed to rise to the top as cream.
Heterogeneous Mixtures
Suppose there is a mixture of white sugar and white sand. Both substances are solids and difficult to separate. It is also very hard to tell them apart, unlike a package of different-colored M &M’s. In some mixtures, all types of matter are solid. In others, such as soda pop, some types are solid, some are liquid, and some are bubbles of gas. Rocks and minerals are also heterogeneous mixtures. Different types of chemical compounds, water, and other solids combine to form them. A diamond is formed of crystalline carbon under conditions of extreme heat and pressure. However, diamonds in the real world contain impurities that affect their color and clarity. Boron is responsible for those that are blue; nitrogen, yellow and brown; and deformation or irradiation for other colors.
Interested in chemistry tutoring services? Learn more about how we are assisting thousands of students each academic year.
SchoolTutoring Academy is the premier educational services company for K-12 and college students. We offer tutoring programs for students in K-12, AP classes, and college. To learn more about how we help parents and students in Winnipeg, MB, Canada: visit Tutoring in Winnipeg, MB, Canada