An atom is a basic building block of matter around us. Each atom is consists of the fundamental particles. Electron is negatively charged, proton is positive and neutron is neutral. Electrons revolve around positively charged nucleus which is made of protons and neutrons.
Atomic symbol
The atoms of all the elements are represented by a unique atomic symbol which is a one or two lettered notation used to represent an atom corresponding to an element. If the atomic symbol has two letters, the first letter is capitalized and the second is in lower case.
Hydrogen is represented by the letter H.
Nitrogen is represented by the letter N.
Helium is represented by the letter He.
Lithium is represented by the letter Li.
Each element has a unique symbol.
Atomic Number and Atomic mass
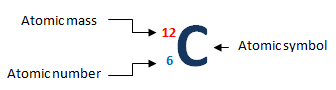
The atomic number of an atom is the number of protons in its nucleus. Each element has a unique atomic number that identifies the number of protons. For example, atomic number of hydrogen is 1 which means it contains only 1 proton in its nucleus. Similarly, carbon has 6 protons in its nucleus which implied atomic number of a Carbon atom is 6
The atomic mass (weight), also called as mass number, is the sum of protons and neutrons in the nucleus. For example, mass number of carbon is 12 which mean it has total number of protons and neutrons in the nucleus.
The atom of an element can be represented as:
Atomic number = number of protons
Atomic mass = number of protons + number of neutrons
An atom is neutral due to equal number of electrons in the orbits and protons in the nucleus. If we know atomic number, we get to know the number of electrons also.
SchoolTutoring Academy is the premier educational services company for K-12 and college students. We offer tutoring programs for students in K-12, AP classes, and college. To learn more visit SchoolTutoring Academy.com.