Electron configuration was first conceived of under the Bohr model of the atom. Electrons revolve around the positively charged nucleus (made of neutrons and protons) in orbits called shells. Each shell can hold up to 2n2 electrons, where n is the shell number. The shell closest to nucleus is called the ‘K shell’ (also called ‘1 shell), followed by ‘L shell’ (or ‘2 shell’), then ‘M shell’ (or ‘3 shell’) and so on. The K shell can hold up to 2 electrons, the L shell can hold up to 8 electrons, the M shell can occupy up to 18 electrons.
Each shell is composed of one or more subshells. A subshell is the set of states defined by azimuthal quantum number, l, within a shell. The values l = 0, 1, 2, 3 correspond s, p, d and f subshells, respectively. The maximum number of electrons which can occupy a subshell is given by 2(2l + 1). This gives two electrons in an s subshell, six electrons in a p subshell, ten electrons in a d subshell and fourteen electrons in an f subshell. The first K shell has one subshell, called ‘1s’; the L shell has two subshells, called ‘2s’ and ‘2p’; the third shell has ‘3s’, ‘3p’, and ‘3d’; and so on.
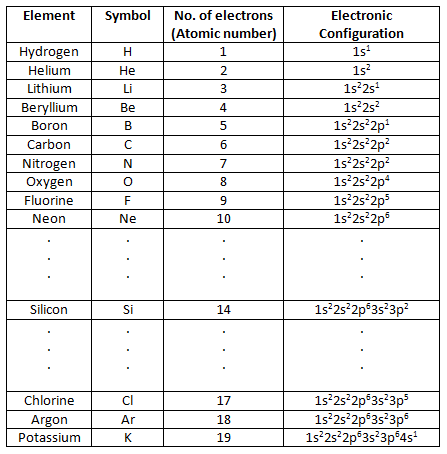
The table below shows electronic configuration for some elements.
SchoolTutoring Academy is the premier educational services company for K-12 and college students. We offer tutoring programs for students in K-12, AP classes, and college. To learn more about how we help parents and students, visit: SchoolTutoring Academy.