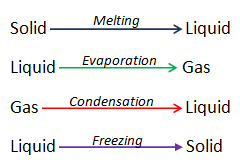
Water covers about 70% of the earth’s surface, being the most abundant compound on the planet. It is tasteless, odorless and colorless. It is very commonly referred as universal solvent as many substances dissolve in it. Water exists in all three states; solid, liquid and gaseous. Water changing from solid to liquid is said to be melting. When it changes from liquid to gas it is called evaporation. Water changing from gas to liquid is called condensation. Water has some unique properties described below.
Cohesion and Adhesion
The hydrogen bond between water molecules is the reason behind properties: cohesion and adhesion. Cohesion refers to the fact that water sticks to itself very easily due to the collective action of hydrogen bonds between water molecules. These hydrogen bonds are constantly breaking and new bonds are forming with different water molecules; but at any given time in a sample of liquid water, a large portion of the molecules are held together by such bonds.
Adhesion means that water also sticks very well to other things. When water comes into contact with these surfaces, the adhesive forces are stronger than the cohesive forces, which is why it spreads out in a thin film on certain surfaces, like glass.
Surface Tension
This property explains the round shape of water drops. The molecules on the surface of the water are not surrounded by similar molecules on all sides, so they’re being pulled deep inside only by cohesion from other molecules deep inside. These molecules cohere to each other strongly but adhere to the other medium weakly. This is the way the water beads up on waxy surfaces. Surface tension makes these water drops round so they cover the smallest possible surface area.
Capillary Action
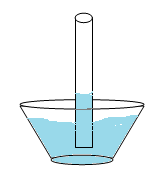
Capillary action is related to the adhesive force and surface tension. If a narrow tube is placed in a container of water the level of water rises in the tube against the force of gravity. Water adheres to the inside wall of the tube and surface tension tends to straighten the surface causing a surface rise and more water is pulled up through cohesion. The process continues as the water flows up the tube until there is enough water such that gravity balances the adhesive force. This happens in plants when they “suck up” water. The water adheres to the inside of the plant’s tubes, but the surface tension attempts to flatten it out. This makes the water rise and cohere to itself again, a process that continues until enough water builds up to make gravity begin pulling it back down.
SchoolTutoring Academy is the premier educational services company for K-12 and college students. We offer tutoring programs for students in K-12, AP classes, and college. To learn more about how we help parents and students in Pembroke visit: Tutoring in Pembroke.