Chemistry isn’t restricted to things that happen in the lab. In fact, everything on this planet is made up of atoms – the same 118 (or more) elements that are found on the periodic table. Your first encounter with the periodic table can be quite daunting at first, in fact most students find it quite overwhelming. With such an large amount of information that is presented, students often forget and mix up all the information that is presented on the table. However, with a better understanding of the information given to us on the periodic table, approaching chemistry problems can be easier than ever.
 The periodic table is assembled into various rows and columns. Each row of the periodic table is referred to as a period, and all elements in the same row contain the same number of atomic orbitals (i.e the amount of “space” electrons can be found in). If you look at the 3rd row of the periodic table for example, all elements that are aligned in that same row will all contain the same number of atomic orbitals. On the other hand each column of the periodic table is called a group and all elements that fall in the same column contain the same amount of valence electrons (electrons present on the other most outer atomic orbital). These electrons are very important as they are the ones interact and create bonds with other elements on the periodic table.
The periodic table is assembled into various rows and columns. Each row of the periodic table is referred to as a period, and all elements in the same row contain the same number of atomic orbitals (i.e the amount of “space” electrons can be found in). If you look at the 3rd row of the periodic table for example, all elements that are aligned in that same row will all contain the same number of atomic orbitals. On the other hand each column of the periodic table is called a group and all elements that fall in the same column contain the same amount of valence electrons (electrons present on the other most outer atomic orbital). These electrons are very important as they are the ones interact and create bonds with other elements on the periodic table.
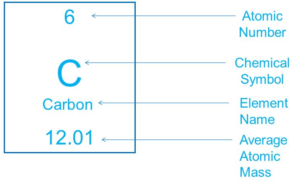
Now, let’s take a look into the information given about a single element on the periodic table. Let’s take a look carbon for example.
Atomic Number
The atomic number of an element tells you several pieces of information about the element. First off the atomic number tells you the order it presents itself in the periodic table. Looking at the example above, the atomic number of carbon is 6 so it is the 6th element found on the periodic table. In addition to this the number 6 tells you the total number of protons found in the nucleus of this element and the total number of electrons found in its atomic orbitals
Chemical Symbol
This is often a one or two letter abbreviation of the element’s name. In the case above, the chemical symbol for carbon is simply just the letter “C”
Element Name
This is the common name of the element and what it is generally referred to as.
(Average) Atomic Mass
The atomic mass or average atomic mass of an element is the average mass of all the found isotopes of the element. The number that is written on the periodic table is the weight of the most common isotope found in nature or in the lab.
SchoolTutoring Academy is the premier educational services company for K-12 and college students. We offer tutoring programs for students in K-12, AP classes, and college. To learn more about how we help parents and students in Houston, Texas visit: Tutoring in Houston, Texas.