Overview
The part of chemistry that deals with matter is concerned with the world of objects that have mass and take up space. A material object may be in solid, liquid, or gaseous form; consist of pure substances or mixtures of pure substances; contain elements or compounds; and may be composed of metal, nonmetal, or metalloid.
States of Matter
A material object can be classified according to its state. If it is a solid, it has a definite shape and volume. The shape of a solid is not dictated by its container. Suppose you have a solid marble, hard, spherical, and smooth. It will be the same size and shape whether it is immersed in water, on the edge of a table, or lost in the bushes outside. By contrast, the molecules of a liquid move much more freely and take the shape of their container. They do have a definite volume. Gases have indefinite volume and indefinite shape. They expand and contract under pressure.
Pure Substances or Mixtures
A material object also exists as a pure substance or a mixture of various substances. Suppose an ingot of gold is refined until it is 24-carat pure gold, and then it is refined more, until as many atoms as possible are gold. The more pure gold is, the softer it is, so jewelers alloy gold with other substances to make it more useful. Rose gold is a mixture of gold and copper, and white gold is a mixture of palladium, silver, or titanium with gold.
Elements or Compounds
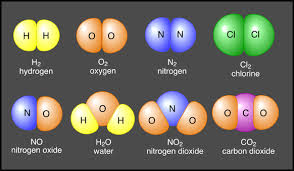
Matter may also exist as one of the 109+ elements in the periodic table. Some, such as carbon (C), are very common, while others are very rare. The heaviest elements have been produced in minute quantities in scientific laboratories, and decay rapidly into other elements. Compounds consist of elements joined with various types of chemical bonds. Water, H2O, is an example of a compound. Each molecule contains 2 atoms of hydrogen and 1 atom of oxygen.
Metal, Nonmetal, or Metalloid
Elements can be classified further as metal, nonmetal, or metalloid, and have been arranged in the periodic table on the basis of their properties. Most of the elements are metals, which are usually solid at room temperature, lustrous, good conductors, malleable, and ductile. Nonmetals may be solids, liquid, or gaseous at room temperature, not lustrous, poor conductors, and brittle. Metalloids share some properties of metals and some of nonmetals. Some nonmetals are highly reactive with other elements.
Interested in chemistry tutoring services? Learn more about how we are assisting thousands of students each academic year.
SchoolTutoring Academy is the premier educational services company for K-12 and college students. We offer tutoring programs for students in K-12, AP classes, and college. To learn more about how we help parents and students in Halifax, NS, Canada: visit Tutoring in Halifax, NS, Canada