Bohr-Rutherford diagrams are simple atomic models that show the number of electrons in each shell of an atom. While they are a major simplification of what really happening in an atom, they can be useful to help with visualizing electrons orbiting a nucleus. Drawing Bohr-Rutherford diagrams is super easy using the following steps:
- Find the number of protons, neutrons and electrons for the atom. The number of protons is the atomic number. The number of neutrons can be found by subtracting the number of protons from the atomic mass rounded to the nearest whole. This is because protons and neutrons both weigh 1 atomic mass unit (amu) and electrons weigh essentially 0 amu. To find the number of electrons you have to compare the charge to the number of protons. A neutral atom will have the same number of electrons as protons. For cations (positive ions), the number of electrons will equal the number of protons minus the charge. For anions (negative ions), the number of electrons will equal the number of protons plus the absolute value of the charge. This is because protons are positively charged and electrons are negatively charged so cations will have more protons than electrons and vice versa for anions.
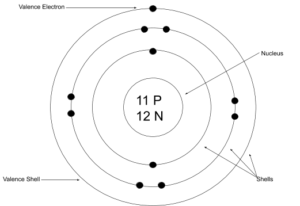
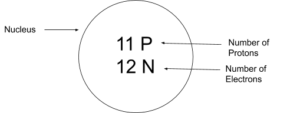
- Set up the diagram. To set up the diagram, you will need a circle in the middle. This will represent the nucleus. Here you will write the number of protons and neutrons as shown below in this example using sodium (Na)
- Add in orbitals and electrons. In the last step you will need to draw circles around the nucleus. These will represent the shells in which the electrons orbit the nucleus. Two electrons will go in the first shell closest to the nucleus and eight can go in each subsequent shell. Using the example of sodium started above, we know that sodium as a neutral atom will have 11 electrons. That means two electrons will go in the first shell, eight in the second and one in the third. That third orbital is our outer shell or valence shell, meaning that sodium has one valence electron.