Atoms. What are they? If you are taking Chemistry then you should know that they are the basic building blocks of all matter. You may have also learnt that the atom is made up of sub-atomical particles known as protons, neutrons and electrons. But did you know that the model of an atom has changed many times over the centuries?
The story of the atom begins way back in the days of the Greek philosopher Democrotus (470-380 BCE) who proposed that all matter was made up of smaller particles he dubbed “atomos”.
It wasn’t until 1808 before an English school teacher, named John Dalton, would use experiments to elaborate on these Atoms. He created the first Atomic theory which consisted of these main points:
-
- All matter is made up of tiny, indivisible particles he called atoms. He imagined them as hard with a distinct mass and movable. They could neither be created nor destroyed.
- All atoms of the same element are identical. Identical in terms of mass and also properties.
- Atoms of different elements are different.
- Compounds are formed by combining atoms of different elements in fixed whole numbered ratios.
- Chemical reactions occur when atoms are rearranged.
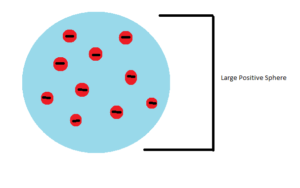
In 1897 J.J. Thompson proved Dalton wrong when he discovered electrons. This showed that the atoms were not indivisible as Dalton had suggested. Thompson did experiments using Cathode Rays which were thought to be beams of atoms at the time. He noticed that the beam travelled further through air than their size would suggest. He was able to estimate that the beam was made up of particles that were 1000 times lighter than a Hydrogen atom. Further experiments using electric fields to deflect the ray showed that the particle was negatively charged and the same in every type of element. With these results he proposed the “Plum Pudding” model of the atom. This model suggested that atoms were a large positive sphere with negative electrons embedded into it.
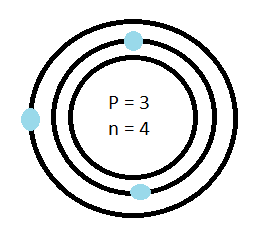
Earnest Rutherford discovered protons and the nucleus in 1911. In his experiment he shot a beam of positive particles at gold foil. He found that most of the particles would go straight through the particle but strangely a couple would be deflected. He proposed that rather than the atom being a large positive sphere, as Thompson had suggested, the atom had a small dense center (nucleus) with positive charges. The positive charges were surrounded mostly by empty space with electrons orbiting around the nucleus. Neil Bohr expanded on this idea by proposing that the electrons orbited the nucleus in levels which he called shells.
In 1920 Rutherford realized there was a discrepancy between the mass of the atoms and the mass between the combined protons and electrons. He thought that there must be another particle that was neutral in charge which he would call a Neutron. He asked James Chadwick to find evidence for this Neutron. Later in his life, Chadwick became interested in experiments where Beryllium was bombarded by Alpha particles. The hit Beryllium would emit radiation with strange properties. The strange beam was able to knock loose protons. Chadwick, after confirming with his own experiments, concluded that the beam was made up of a neutral particle with the same mass as a proton.
Combining all these previous discoveries and proposals we can see the model of the atom as the Bohr-Rutherford model.
In modern days, there have been even more new developments on the model of the atom. For example, we know that the nucleus is extremely dense and tiny compared to the rest of the atom and that the majority of the atom’s mass lies within the nucleus. Also, rather than in rings, the electrons actually move around the nucleus in electron clouds. However, electrons are so small that this is mostly empty space. Because the electrons are so small, it is also impossible for us to tell where an electron is at any point of time. Instead, we can only determine the probability of an electron to be in a specific area.
As we learn more about the atom, the atomic model may change again and again. We are constantly learning new things in science and tweaking the models used to represent our current knowledge.