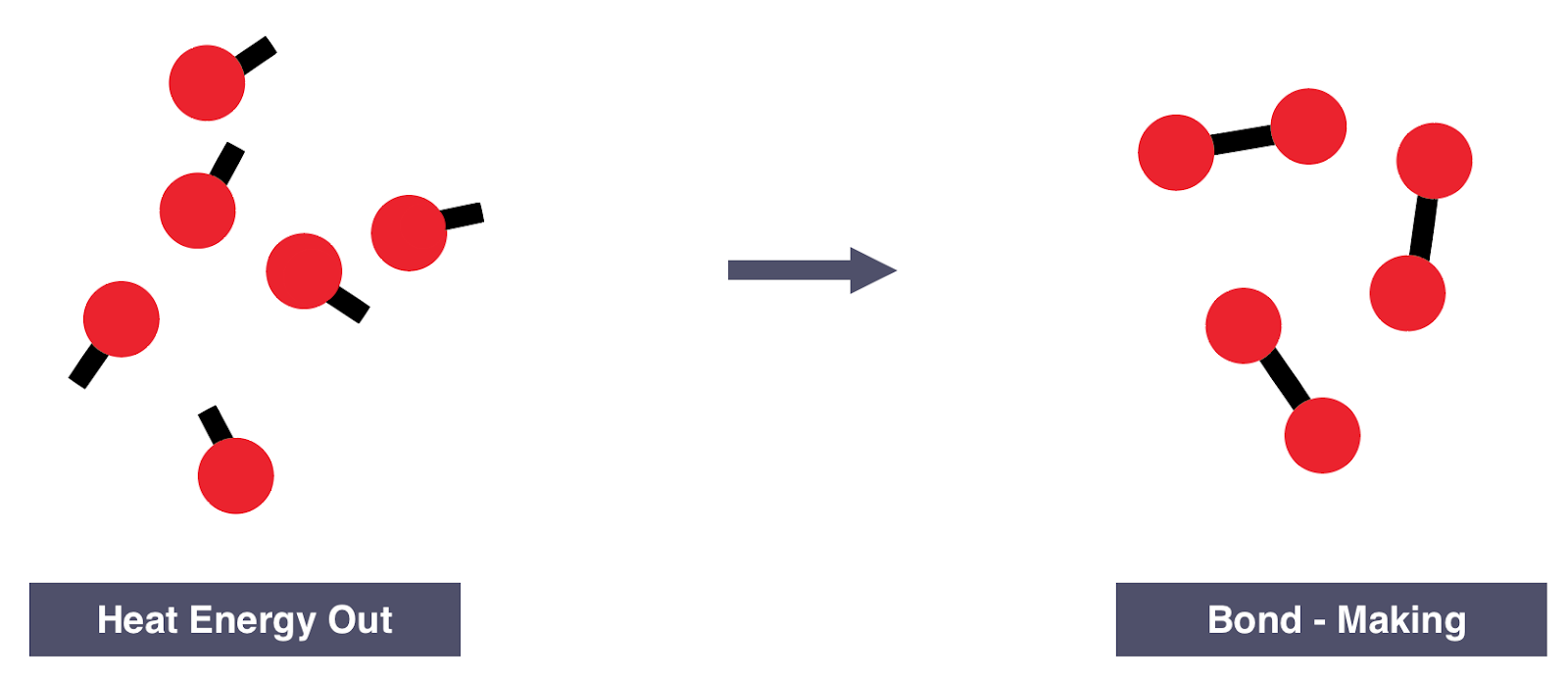
All molecules are held together by bonds. These bonds come in different lengths, strengths, and numbers, but they all share one thing in common: energy. All bonds store energy which…
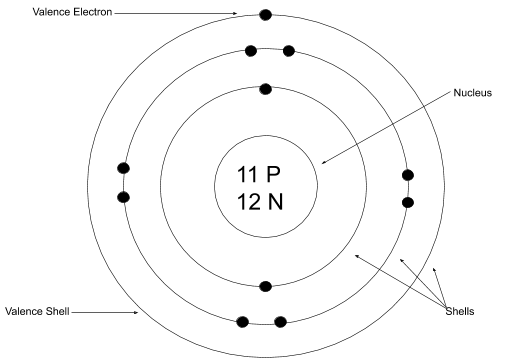
read moreBohr-Rutherford diagrams are simple atomic models that show the number of electrons in each shell of an atom. While they are a major simplification of what really happening in an…
read moreAtoms. What are they? If you are taking Chemistry then you should know that they are the basic building blocks of all matter. You may have also learnt that the…
read moreA difficult concept in chemistry is the equilibrium of chemical equations. Chemical reactions do not always react to completion, and they can proceed in either direction if it is written…
read moreThere are various types of chemical reactions that occur in chemistry, but many reactions tend to follow 1 of 5 main types. Becoming familiar with these types of chemical reactions…
read moreChemistry isn’t restricted to things that happen in the lab. In fact, everything on this planet is made up of atoms – the same 118 (or more) elements that are…
read moreOften in chemistry chemical reactions are written in an equation form using chemical symbols. The reactants of the chemical reactants are placed on the left hand side of the reaction…
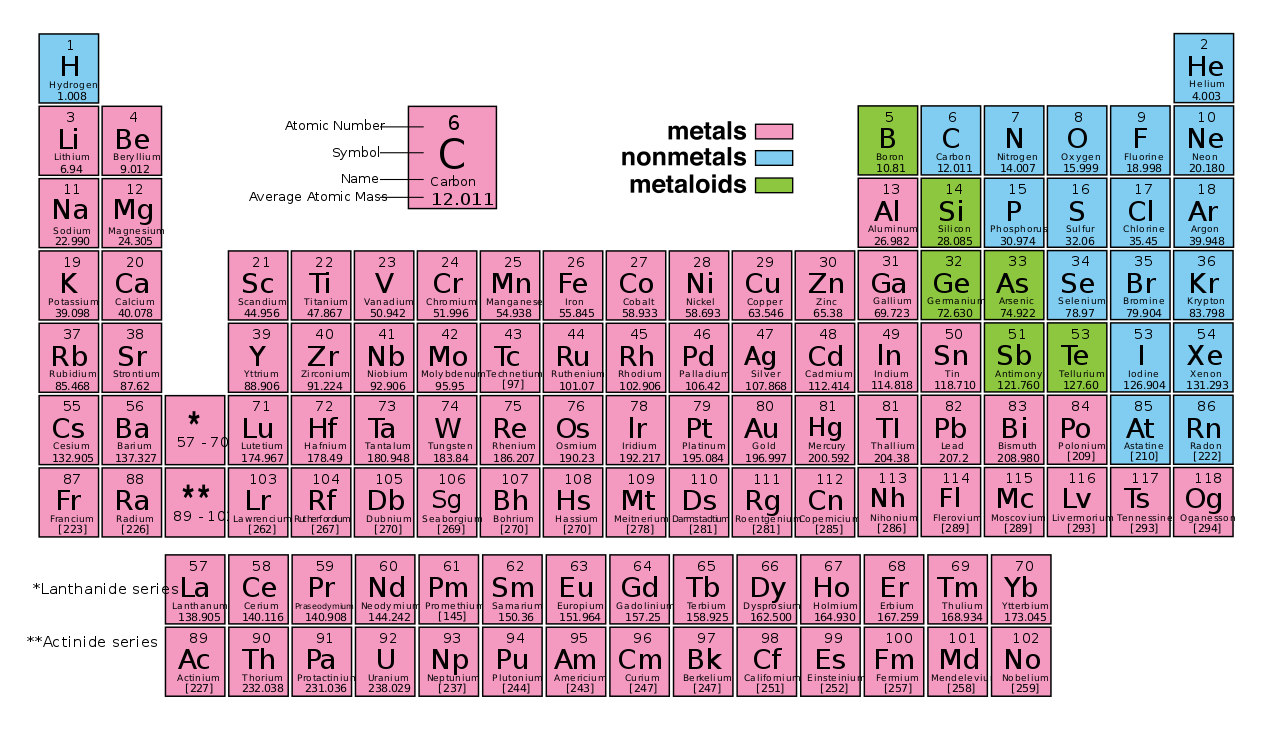
read moreOverview Metalloids are elements that have some properties of metals and some of nonmetals. They are on the periodic table along the dividing line between metals and nonmetals. The most…
read moreOverview The element mercury (Hg) has atomic number 80, and is considered a transition metal. It is liquid at room temperature. Although mercury is highly toxic, it is a good…
read moreOverview The transition metals occupy a large area on the periodic table. They all appear to have similar metallic properties, because of the way their electron shells are filled. Many…
read more