Overview
Most elements have isotopes, but not all isotopes are radioactive. Many isotopes are stable variations in mass, occurring naturally. Unstable isotopes decay into other isotopes and elements, and emit energy which is picked up as radioactivity.
Isotopes
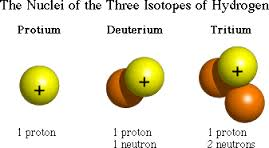
An element is defined by the number of protons it has in its atomic nucleus. For example, the element hydrogen has one proton in its nucleus. It has three different isotopes: hydrogen-1, with one proton and no neutrons in its nucleus; hydrogen-2 (also called deuterium) with one proton and one neutron in its nucleus, and hydrogen-3 (also called tritium), with one proton and 2 neutrons. The number of neutrons determine the type of isotope it is. Similarly, the element oxygen has three stable isotopes, all with 8 protons in its atomic nucleus. Oxygen-16 has 8 neutrons, oxygen-17 has 9 neutrons, and oxygen-18 has 10 neutrons.
|
Figure 1: Isotopes differ in the number of neutrons in the atom |
Radioactive Isotopes
An element is radioactive if it is unstable and decays into other elements, emitting subatomic particles and energy. Radioactive elements that occur in nature take so long to decay that they still exist as elements. For example, the element uranium is naturally radioactive. One of its isotopes, Uranium-235, has 92 protons and 143 neutrons in its atomic nucleus. Another isotope, Uranium-238 has 92 protons and 146 neutrons in its atomic nucleus. Other elements are not radioactive naturally have radioactive isotopes that can be produced artificially.
The Half-Life
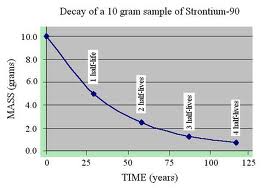
For example, it takes 4.5 x 109 years for Uranium-238 to decay, and 7.1 x 108 years for Uranium 235 to decay. Other radioisotopes have much shorter half-lives. The radioisotope Cesium-137 has a half-life of 30 years. Starting with a 10 gram sample today, 5 grams would be left in 30 years, 2.5 grams would be left in 60 years, and 1.25 grams in 90 years.
|
Figure 2: Example of the measurement of radioactive decay in the number of half-lives. |
Carbon Dating
The element carbon exists in all living things, as well as things that once were living. One of its isotopes, Carbon-14, naturally radioactive, with a half-life of about 5730 years. In radiocarbon dating, the ratio of Carbon-14 to the other naturally occurring isotopes of carbon, is measured, so the approximate age of the item can be deduced. The paper of the Dead Sea Scrolls, wood samples from the tombs of Egyptian kings, and prehistoric skeletal remains also some of the many objects that have been studied to estimate their age.
Interested in chemistry tutoring services? Learn more about how we are assisting thousands of students each academic year.
SchoolTutoring Academy is the premier educational services company for K-12 and college students. We offer tutoring programs for students in K-12, AP classes, and college. To learn more about how we help parents and students in Rochester, NY: visit Tutoring in Rochester, NY