Overview
The element hydrogen is the lightest of all elements in the periodic table, with one proton in its nucleus. It is also the most abundant element in the universe. Hydrogen has 3 naturally occurring isotopes, although others have been produced experimentally.
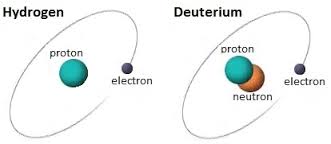
Hydrogen
The most common form of hydrogen has one proton in its nucleus and no neutrons. It occurs over 99% of the time, often in common compounds on Earth such as H2O, or water. At room temperature, hydrogen gas itself is colorless, odorless, highly reactive, and flammable. It forms explosive reactions with other elements such as the oxygen in the air and chlorine.
Deuterium
Deuterium is a stable isotope of hydrogen, containing one proton and one neutron in its nucleus. It is twice as heavy as the common form of hydrogen. Most often on Earth, highly concentrated deuterium occurs in a form of water called “heavy water”, because water containing deuterium atoms is heavier and more viscous than ordinary water. For example, ice made of heavy water sinks rather than floats in regular water. Since some occurs naturally, it can be separated out by various processes and is often used commercially to cool nuclear reactors.
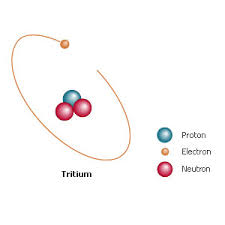
Tritium
The tritium atom contains one proton and 2 neutrons. It occurs naturally in the upper atmosphere by cosmic rays striking hydrogen molecules. Like deuterium, some tritium atoms find their way into chemical reactions with oxygen to form water. However, tritium is radioactive, with a half-life of about 12.32 years. Its natural decay gives scientists a way to measure the age of groundwater, since some water containing tritium falls as rain. Water that has fallen over 100 years ago contains no radioactive tritium, but more recent rainfall does.
Hydrogen in Space
Free hydrogen exists in interstellar clouds. Most stars consist of hydrogen plasma, used in nuclear fusion reactions. Deuterium has been found in the solar corona, spectroscopic observations of other stars, as well as in the atmospheres of Jupiter and Venus. Some comets, such as Halley’s Comet and Comet Hale-Bopp, have a high proportion of deuterium. Scientists theorize that some of the water on Earth may have come from comets and asteroids striking its surface.
Interested in science tutoring services? Learn more about how we are assisting thousands of students each academic year.
SchoolTutoring Academy is the premier educational services company for K-12 and college students. We offer tutoring programs for students in K-12, AP classes, and college. To learn more about how we help parents and students in Kingston, ON, Canada: visit Tutoring in Kingston, ON